top of page

PHYSICAL SCIENCE
Subshells
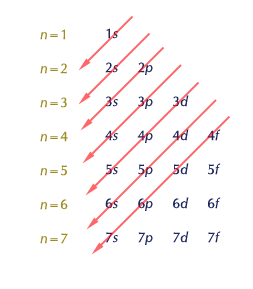
Each shell is composed of one or more subshells, which are themselves composed of atomic orbitals.
For example, the first (K) shell has one subshell, called 1s; the second (L) shell has two subshells, called 2s and 2p; the third shell has 3s, 3p, and 3d; the fourth shell has 4s, 4p, 4d and 4f;
bottom of page